


























































































Prepara tus exámenes y mejora tus resultados gracias a la gran cantidad de recursos disponibles en Docsity

Gana puntos ayudando a otros estudiantes o consíguelos activando un Plan Premium


Prepara tus exámenes
Prepara tus exámenes y mejora tus resultados gracias a la gran cantidad de recursos disponibles en Docsity
Prepara tus exámenes con los documentos que comparten otros estudiantes como tú en Docsity
Los mejores documentos en venta realizados por estudiantes que han terminado sus estudios
Estudia con lecciones y exámenes resueltos basados en los programas académicos de las mejores universidades
Responde a preguntas de exámenes reales y pon a prueba tu preparación

Consigue puntos base para descargar
Gana puntos ayudando a otros estudiantes o consíguelos activando un Plan Premium
Comunidad
Pide ayuda a la comunidad y resuelve tus dudas de estudio
Descubre las mejores universidades de tu país según los usuarios de Docsity
Ebooks gratuitos
Descarga nuestras guías gratuitas sobre técnicas de estudio, métodos para controlar la ansiedad y consejos para la tesis preparadas por los tutores de Docsity
USMLE Road Map Bioquímica
Tipo: Apuntes
1 / 236

Esta página no es visible en la vista previa
¡No te pierdas las partes importantes!





























































































LANGE
LANGE
RICHARD G. MACDONALD
Department of Biochemistry and Molecular Biology University of Nebraska Medical Center Omaha, Nebraska
WILLIAM G. CHANEY
Department of Biochemistry and Molecular Biology University of Nebraska Medical Center Omaha, Nebraska
New York Chicago San Francisco Lisbon London Madrid Mexico City Milan New Delhi San Juan Seoul Singapore Sydney Toronto
Copyright © 2007 by The McGraw-Hill Companies, Inc. All rights reserved. Manufactured in the United States of America. Except as permitted under the United States Copyright Act of 1976, no part of this publication may be reproduced or distributed in any form or by any means, or stored in a database or retrieval system, without the prior written permission of the publisher.
0-07-159319-
The material in this eBook also appears in the print version of this title: 0-07-144205-7.
All trademarks are trademarks of their respective owners. Rather than put a trademark symbol after every occurrence of a trademarked name, we use names in an editorial fashion only, and to the benefit of the trademark owner, with no intention of infringement of the trademark. Where such designations appear in this book, they have been printed with initial caps.
McGraw-Hill eBooks are available at special quantity discounts to use as premiums and sales promotions, or for use in corporate training programs. For more information, please contact George Hoare, Special Sales, at george_hoare@mcgraw-hill.com or (212) 904-4069.
TERMS OF USE
This is a copyrighted work and The McGraw-Hill Companies, Inc. (“McGraw-Hill”) and its licensors reserve all rights in and to the work. Use of this work is subject to these terms. Except as permitted under the Copyright Act of 1976 and the right to store and retrieve one copy of the work, you may not decompile, disassemble, reverse engineer, reproduce, modify, create derivative works based upon, transmit, dis- tribute, disseminate, sell, publish or sublicense the work or any part of it without McGraw-Hill’s prior consent. You may use the work for your own noncommercial and personal use; any other use of the work is strictly prohibited. Your right to use the work may be terminated if you fail to comply with these terms.
THE WORK IS PROVIDED “AS IS.” McGRAW-HILL AND ITS LICENSORS MAKE NO GUARANTEES OR WARRANTIES AS TO THE ACCURACY, ADEQUACY OR COMPLETENESS OF OR RESULTS TO BE OBTAINED FROM USING THE WORK, INCLUDING ANY INFORMATION THAT CAN BE ACCESSED THROUGH THE WORK VIA HYPERLINK OR OTHERWISE, AND EXPRESSLY DISCLAIM ANY WARRANTY, EXPRESS OR IMPLIED, INCLUDING BUT NOT LIMITED TO IMPLIED WARRANTIES OF MERCHANTABILITY OR FITNESS FOR A PARTICULAR PURPOSE. McGraw-Hill and its licensors do not warrant or guarantee that the functions contained in the work will meet your requirements or that its operation will be uninterrupted or error free. Neither McGraw-Hill nor its licensors shall be liable to you or anyone else for any inaccuracy, error or omission, regardless of cause, in the work or for any damages resulting therefrom. McGraw-Hill has no responsibility for the content of any information accessed through the work. Under no circumstances shall McGraw-Hill and/or its licensors be liable for any indirect, incidental, special, punitive, consequential or similar damages that result from the use of or inability to use the work, even if any of them has been advised of the possibility of such damages. This limitation of liability shall apply to any claim or cause whatsoever whether such claim or cause arises in contract, tort or otherwise.
DOI: 10.1036/
vi Contents
Contents vii
U S I N G T H E
U S M L E R O A D M A P S E R I E S
F O R S U C C E S S F U L R E V I E W
What is the Road Map Series?
Short of having your own personal tutor, the USMLE Road Map Series is the best source for efficient review of major concepts and information in the medical sciences.
Why Do You Need A Road Map?
It allows you to navigate quickly and easily through your biochemistry and genetics course notes and textbook and prepares you for USMLE and course examinations.
How Does the Road Map Series Work?
Outline Form: Connects the facts in a conceptual framework so that you understand the ideas and retain the in- formation.
Color and Boldface: Highlights words and phrases that trigger quick retrieval of concepts and facts.
Clear Explanations: Are fine-tuned by years of student interaction. The material is written by authors selected for their excellence in teaching and their experience in preparing students for board examinations.
Illustrations: Provide the vivid impressions that facilitate comprehension and recall.
Clinical Correlations: Link all topics to their clinical applications, promoting fuller understanding and memory retention.
Clinical Problems: Give you valuable practice for the clinical vignette-based USMLE questions.
Explanations of Answers: Are learning tools that allow you to pinpoint your strengths and weaknesses.
ix
CORRELATION^ CLINICAL
C O M M O N A B B R E V I A T I O N S
ADP adenosine diphosphate
AMP adenosine monophosphate
ATP adenosine triphosphate CNS central nervous system
FAD flavin adenine dinucleotide (oxidized form)
FADH 2 flavin adenine dinucleotide (reduced form)
GDP guanosine diphosphate GMP guanosine monophosphate
GTP guanosine triphosphate
HDL high-density lipoprotein
LDL low-density lipoprotein NAD+^ nicotinamide adenine dinucleotide (oxidized form)
NADH nicotinamide adenine dinucleotide (reduced form)
NADP+^ nicotinamide adenine dinucleotide phosphate (oxidized form)
NADPH nicotinamide adenine dinucleotide phosphate (reduced form) Pi inorganic orthophosphate
PPi inorganic pyrophosphate
RBC red blood cell
VLDL very low-density lipoprotein WBC white blood cell
x
Copyright © 2007 by The McGraw-Hill Companies, Inc. Click here for terms of use.
A. The special chemical properties of water make it ideal as the main physiologic sol- vent for polar substances in the body.
1. Within the water molecule, the oxygen nucleus draws electrons away from the hydrogen atoms, producing an internal charge separation that makes each mol- ecule magnetic or polar. 2. Substances that dissolve well in water are referred to as polar or hydrophilic. 3. Molecules that dissolve sparingly in water are nonpolar or hydrophobic. B. Water molecules bind with each other through important noncovalent interac- tions called hydrogen bonds. 1. Hydrogen bonds result from attraction between the partially positively charged hydrogen atoms of one molecule and the electronegative atom, usually oxygen or nitrogen, of another molecule. 2. Hydrogen bonds are weak and rapidly break and re-form up to 10 12 times per second in water at 25°C. C. The hydrogen bond network of water molecules confers special properties on water that are important for sustaining life. 1. Water has a high surface tension where it comes in contact with air. a. Surface tension is the force acting to push together the liquid molecules at an air-liquid interface. b. This property causes the liquid to form droplets and to resist passage of sub- stances across the interface. c. The surface tension of fluid at the alveolar air-water interface of the lungs contributes to elastic recoil that causes the alveoli to return to the original volume after inflation during breathing. 2. Water has a high heat of vaporization, ie, the amount of heat needed to con- vert from liquid to gas phase. In conjunction with its high heat capacity, this property allows water to carry away heat efficiently as it evaporates, which ac- counts for the cooling effects of perspiration. 3. Water has a high dielectric constant, which is a measure of its ability to carry electrical current, as it does in nerve cells.
A. Electrolytes are compounds that separate or dissociate in water into a positively charged cation and a negatively charged anion.
C H A P T E RC H A P T E R 11 N
PH Y S I O LO G I C
C H E M I S T RY
1
Copyright © 2007 by The McGraw-Hill Companies, Inc. Click here for terms of use.
2. In pure water, very few water molecules actually undergo this dissociation, and the concentration of water is considered to be a constant, equal to 55.5 M. 3. Reorganization of the equilibrium equation gives a new, combined constant, K w , and the ion product of water.
K w = K eq × 55.5 M = [H+][OH – ]
4. Thus, in pure water, the [H+] = [OH−^ ] = 10−^7 M, and when acid or base is added to water, these ions change concentration in a reciprocal manner. B. The acid state of a solution is represented by its pH, which is calculated from the [H+].
pH = –log[H+]
1. In pure water, where [H+] = 10−^7 M, the pH = 7.0; at this pH, the solution is considered neutral. 2. When the pH is < 7.0, the solution is acidic; when the pH is > 7.0, the solu- tion is basic or alkaline. 3. Human plasma has a pH of 7.4 under normal conditions. a. Maintenance of plasma pH within a narrow range, 7.35 to 7.45, supports the optimal activity of enzymes and function of proteins. b. Deviation of plasma pH from this physiologic range interferes with the function of enzymes and proteins and, therefore, of cells. 4. In contrast, gastric fluid is more acidic (pH = 1.2–2.8) and pancreatic secretion is more alkaline (7.8–8.5).
- Ionized or charged forms of drugs that are weak acids or bases cannot cross biologic membranes _readily because of the nonpolar nature of the lipids that form the membrane bilayer.
A. Solutions of weak acids and bases act as buffers that resist changes in pH when acid or base is added (Figure 1–1). B. The Henderson-Hasselbalch equation is derived from the rearrangement of the equilibrium equation for dissociation of a weak acid.
1. The Henderson-Hasselbalch equation describes the relationship between the pH, the p K a, and the concentrations of the conjugate acid and base. 2. The effectiveness of a buffering system is maximal when it is operating at a pH near its p K a (Figure 1–1). a. When pH ≅ p K a, the buffer is poised to absorb either added H +^ or OH− with minimal change in pH.
pH = p K a + log
[conjugate base]
[conjugate acid]
= p K a + log
Chapter 1: Physiologic Chemistry 3
N
CLINICAL CORRELATION
b. Buffering capacity is also related to the buffer concentration. For exam- ple, the ability of a weak acid solution to buffer added acid is related to the concentration of conjugate base available to combine with the protons. C. The carbonic acid-bicarbonate system is the most important buffer of the blood.
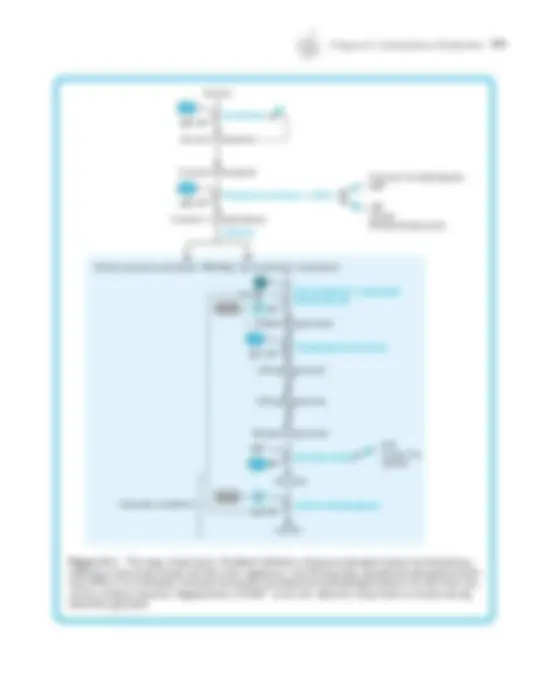
1. Carbonic acid, H 2 CO 3 , is a weak acid that dissociates into a proton and the bicarbonate anion, HCO 3 –^ (Figure 1–2). 2. The carbonic acid-bicarbonate buffer system has a p K a of 6.1, yet is still a very effective buffer at pH 7.4 because it is an open buffer system, in which one component, CO 2 , can equilibrate between blood and air.
CO 2 + H 2 O →← H 2 CO (^3) ←→ H+^ + HCO 3 –
a. This system is very flexible in response to changes in pH of the blood or the peripheral tissues. b. Dissolved CO 2 is in equilibrium with gaseous CO 2 in the alveoli, which allows the lungs to help maintain blood pH by adjusting the amount of CO 2 expired. c. An increase in CO 2 expiration shifts the carbonic acid-bicarbonate equation to the left (decreasing [H+]); a decrease shifts it to the right (increasing [H +]).
3. Dissolved CO 2 can combine with water to form carbonic acid, so CO 2 may be considered an acid from the physiologic standpoint. 4. Bicarbonate ion concentration is regulated mainly by excretion and synthesis in the kidneys.
4 USMLE Road Map: Biochemistry
N
pH = p K a
p K a—1 p K a p K a +
[A—] [HA]
pH
10
1
Buffering zone
Figure 1–1. Weak acids act as buffers in a pH range near their p K as. According to the Henderson-Hasselbalch equation, when the ratio of conjugate base to conjugate acid, [A−^ ]/[HA] is plotted versus pH, a titration curve is generated that indicates a region of good buffering at pH = p K a ± 1 pH unit.
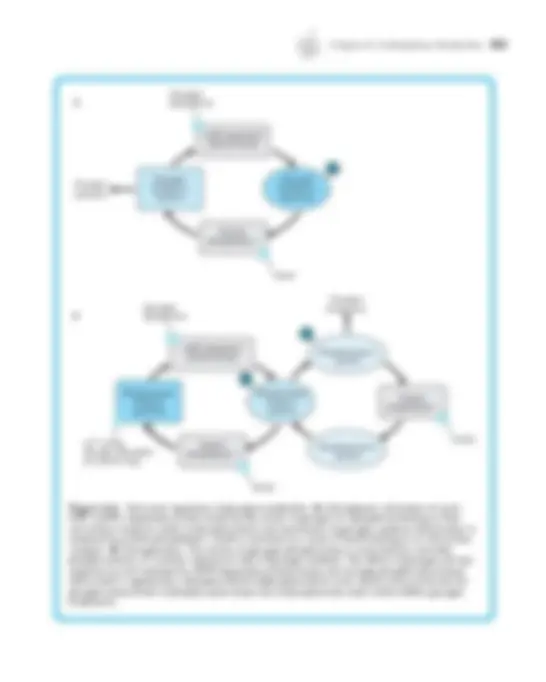
A. Substances that have both a hydrophilic group and a hydrophobic region, often a hydrocarbon tail, are referred to as amphipathic. B. Amphipathic molecules do not dissolve fully in water but instead cluster together to form specialized structures with their polar groups oriented toward the water and nonpolar regions pointed away from the water.
1. Micelles are spherical structures that have the polar groups on the outside sur- face where they form hydrogen bonds with water, and the nonpolar tails are clustered in the core of the structure. 2. An important structure formed by amphipathic molecules is the lipid bilayer, in which the hydrocarbon tails line up in a parallel array with the hydrophilic head groups facing the polar fluids on either side. 3. Lung surfactant is a mixture of proteins and amphipathic lipids that acts like a detergent or soap to greatly decrease the surface tension forces at the alveolar fluid-air interface. a. The main surfactant protein apoprotein SP-A mingles with water molecules to interfere with the hydrogen bond network near the surface. b. The lipid components have their polar head groups inserted into the alveo- lar fluid and hydrophobic tails oriented toward the air.
- The effect of surfactant to reduce the surface tension of the fluid lining the alveoli contributes to de- creased elastic recoil and thereby increases compliance _of the lung.
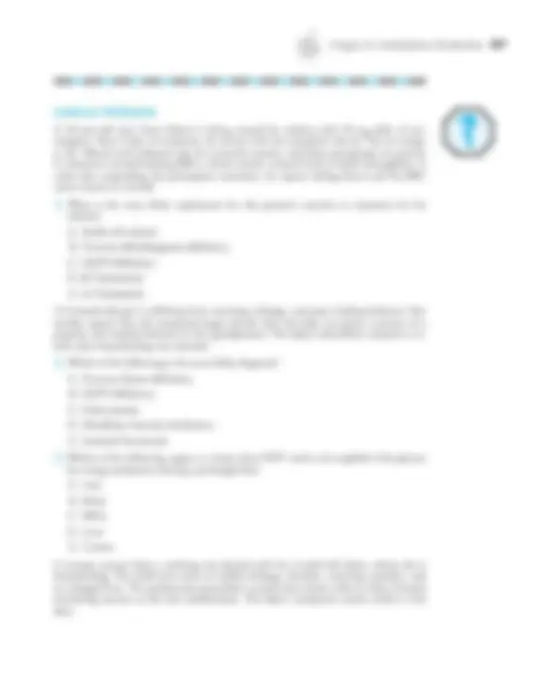
1. The weak organic acid, lactic acid, has a p K a of 3.86. During strenuous exercise, lactic acid can accumulate in muscle cells to produce fatigue. If the ratio of the conjugate base form lactate to the conjugate acid form of lactic acid in muscle cells is approxi- mately 100 to 1, what would be the pH in the muscle cells? A. 1. B. 2. C. 3. D. 4. E. 5.
6 USMLE Road Map: Biochemistry
N
CLINICAL CORRELATION
2. A patient arrives in the trauma center suffering from unknown internal injuries as a re- sult of a traffic accident. She is semiconscious with a blood pressure of 64/40 mm Hg and appears to be going into shock. Blood gases reveal a PCO 2 of 39 mm Hg (normal = 40 mm Hg) and a bicarbonate of 15 mM (normal = 22–30 mM), with pH = 7.22. The best course of action to manage this patient’s acidosis would be to start intravenous ad- ministration of a solution of: A. Sodium bicarbonate B. 5% dextrose C. Sodium lactate D. Sodium hydroxide E. Normal saline 3. Infants born prematurely are at risk for respiratory distress syndrome. In such cases, it is common to administer surfactant, the purpose of which is to alter which of the fol- lowing properties of water at the alveolar interface with air? A. Surface tension B. Evaporation C. Heat of vaporization D. Ionization E. Dielectric constant 4. Lactic acid is considered to be a weak acid because:
A. It is insoluble in water at standard temperature and pressure. B. It fails to obey the Henderson-Hasselbalch equation. C. Little of the acid form remains after it dissolves in water. D. The equilibrium between the acid and its conjugate base has a p K a of 5.2. E. The lactate anion has minimal tendency to attract a proton.
5. The composite p K a of the bicarbonate system, 6.1, may appear to make it ill-suited for buffering blood at physiologic pH of 7.4. Nevertheless, the system is very effective at buffering against additions of noncarbonic acids. Changes in the bicarbonate/carbonic acid ratio in such cases can be regulated by: A. Recruitment of bicarbonate reserves from the peripheral tissues. B. Conversion of carbonic acid to CO 2 and excretion in the urine. C. Conversion of carbonic acid to CO 2 followed by removal by the lungs. D. Reaction of excess carbonic acid with the amino termini of blood proteins. E. Binding of carbonic acid by hydroxide ions from the fluid phase of blood.
Chapter 1: Physiologic Chemistry 7
N